Medical Device Test
Hiller Measurements specializes in developing complex electromechanical and RF test systems for production test of medical devices.
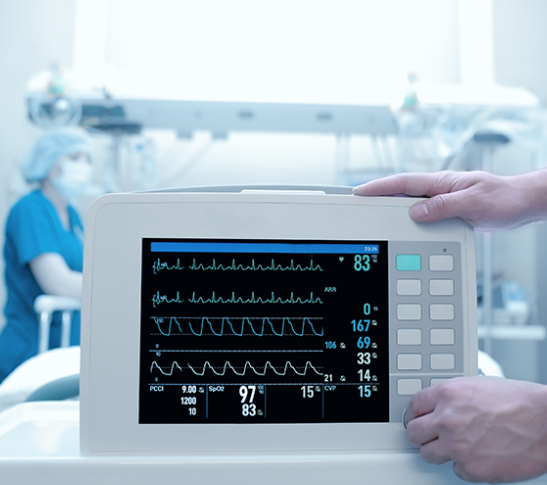
Our Expertise in Highly Specialized Medical Devices Helps Get Products to Market Quickly.
Medical Device Solutions, Powered by Hiller Measurements.

Cardio Rhythmic Management Device RF Test
We offer RF test solutions for implantable cardiac defibrillators, pacemakers, and cardiac resynchronization therapy devices.

Electromechanical Test – Implantable Devices
We develop solutions for testing leads and lead connections for implantable devices.
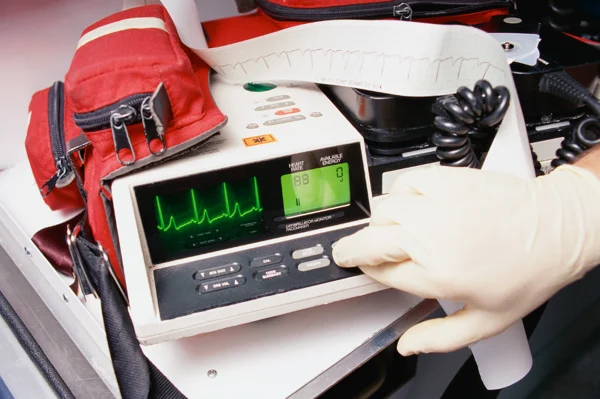
Electronic Test – External Defibrillators
In addition to solutions for implantable devices, we also offer electronic test solutions for external defibrillation devices.

Mission-Critical Power Test
We offer implantable battery test options for mission-critical cardio rhythmic management devices.
Defibrillator Cable Resistance Event Tester
While twisting and bending the leads of a defibrillation device, the Hiller Measurements MPX7-1928 multi-channel event detector and resistance monitoring instrument measures resistance events at a rate of 100us per sample.

Decrease Risk and Reduce Time to Market
Hiller Measurements creates test solutions for medical devices — from implantable devices to ultrasound and MRI equipment, to wearable and subcutaneous medical devices.
By identifying and mitigating risk early in the design process, Hiller Measurements’ flow control process decreases upfront risks as well as the time needed for troubleshooting later in the development process. This combination helps companies get their medical devices to market faster and more safely.

Quality at Speed
We can decrease time to market without sacrificing the confidence that the job is done right.

Regulated Industry Expertise
We have specialized knowledge of the regulatory and business environment for medical devices.

Risk Management
We work with deterministic schedules to ensure production milestones are met with confidence.

Established Partnerships
We have formed trusted teams that are agile and nimble and can change direction quickly if required.

